Arrange the Compounds in Order of Boiling Point.
Pentanol and ethanol have H-bonding but molecular mass of pentanol is more than ethanol so it has high boiling point. Arrange the following compounds in order of increasing boiling point.
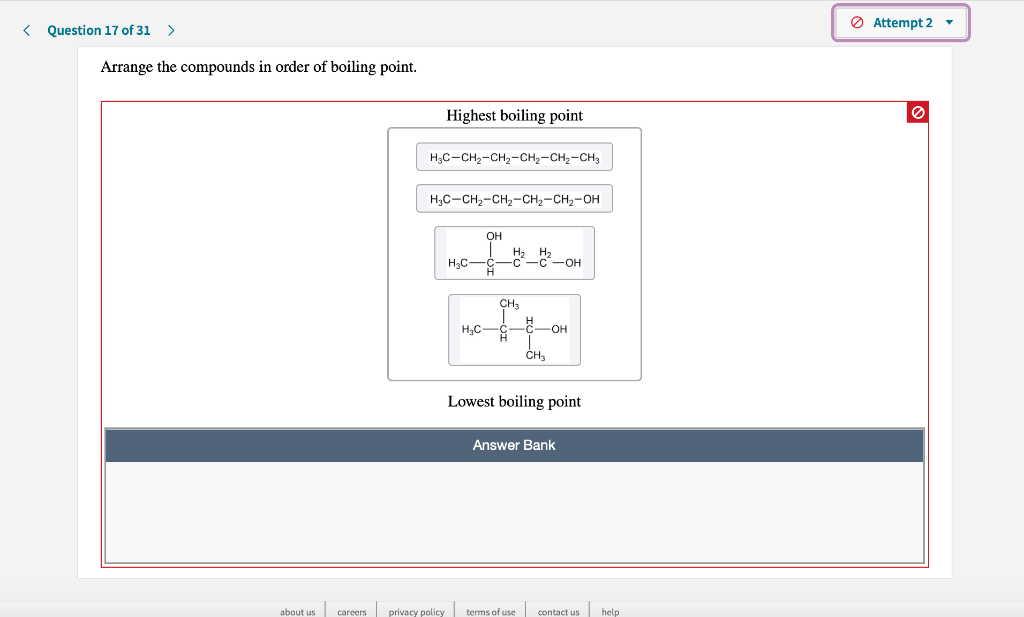
Solved Question 17 Of 31 Attempt 2 Arrange The Compounds Chegg Com
Clear All CH2CH2CH2CH2CCH Lowest boiling point CHCH2CH2CH2CH2CH2CH3 Intermediate boiling point Highest boiling point CH3CH2CH2CH2CH2CH2OH.
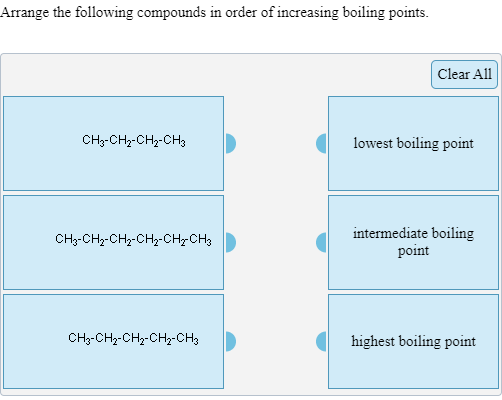
. Part A Arrange the following compounds in order of decreasing boiling point. The boiling point of haloalkanes decreases with an increase in branching. Arrange the following compounds in the increasing order of their boiling points.
Clear All CH3CH2C-OH Lowest boiling point. Part A Arrange the following compounds in order of decreasing boiling point. Arrange the following compounds in increasing order of boiling point.
Therefore the given order would be. A 12-ethanediol n-butane 1-propanol 13-propanediolb CH32CHCH2OH CH32CH CH22CH2OH CH32CH CH23CH2OH ANS. Arrange the following compounds in increasing order of boiling points.
Reset Help Largest Smallest The correct ranking cannot be determined. Therefore the given order would be. A n-butane 1-propanol 12-ethanediol 13-propanediol Compound Molecular weight Number of OH group 13-propanediol 76 12.
Rank from largest to smallest. Arrange the following sets of compounds in order of their increasing boiling points. Arrange the following compounds in increasing order of their boiling pointsAC H 3 C H 2 C H 2 CHOBC H 3 C H 2 C H 2 C H 2 OHCC 2 H 5 O C 2 H 5DC H 3 C H 2 C H 2 C H 3.
Clear A11 CH3CH2CH2CH2CH2CH2CH3 Lowest boiling point CHCHICH CH3CH2CH2CH2CCH3 Intermediate boiling point Highest boiling point CH3CH2CH2CH2C-OH Arrange the following compounds in order of increasing boiling point. A Bromomethane Bromoform Chloromethane Dibromomethane. For questions like these its best to look at the point of difference.
A Pentan-1-ol butan-1-ol butan-2-ol ethanol propan-1-ol me asked Jul 27 2019 in Chemistry by GaneshSahu. Oxygens at the top followed by sulfur followed by the Cellini Ammen and so on. 3 3 -dichloropentane.
Arrange the following compounds in order of increasing boiling point. Q Propanoic acid. Pentanol gt Ethanol gt Propane 3 gt 1 gt 2.
A Pentan-1-ol butan-1-ol butan-2-ol ethanol propan-1-ol methanol. Primary amines RNH2 Secondary amines R2NH Tertiary amines RNR2. For boiling points the increasing order is Hydrocarbons Ethers Aldehyde Alcohol of similar molecular mass.
3 -methyl pentane II. Intermolecular hydrogen bonding is more in primary amines t. B 1-Chloropropane Isopropyl chloride 1.
The decreasing order of boiling points is. Arrange these compounds. C H 3 C H 2 C H 2 C H 2 C H 3 C 2 H 5 O C 2 H 5 C H 3 C H 2.
Arrange the following compounds in increasing order of boiling point. Here we have n. Order of Boiling points of amines.
Propane has the least boiling point due to non-polar character and weak van der Waals force of attration. Arrange each set of compounds in order of increasing boiling points. Rank from largest to smallest.
Thiss question is asking us to rank order three different compounds an increasing boiling point. CO2 CH3OH RbF CH3Br in order of increasing boiling points. Thus the arrangement of the given compounds in the increasing order of their boiling points is given by.
Arrange the following compounds in decreasing order of their boiling pointsBromomethane CH3Br Bromoethane CH3CH2Br Bromoalkane CH3CH2CH2Br Bromobutane CH3CH2CH2CH2Br The Correct Answer is C Bromobutane Bromoalkane Bromoethane Bromomethane. And so the first thing we sort of want to consider when were looking at boiling point is how strong are the inter molecular forces or attractions that hold together the molecules they call these emfs. Propan 1 ol butan 1 ol butan 2 ol pentan 1 ola Propan 1 ol butan 2 ol butan 1 ol pentan 1 ob Propan 1 ol butan 1 ol butan 2 ol pentan 1 olc Propan 1 ol butan 2 ol butan 1 ol pentan 1 old Propan 1 ol butan 1 ol butan 2 ol pentan 1 ol.
CH 3 CH 2 CH 3. Arrange the following sets of compounds in order of their increasing boiling points. Arrange the following compounds in increasing order of their boiling points.
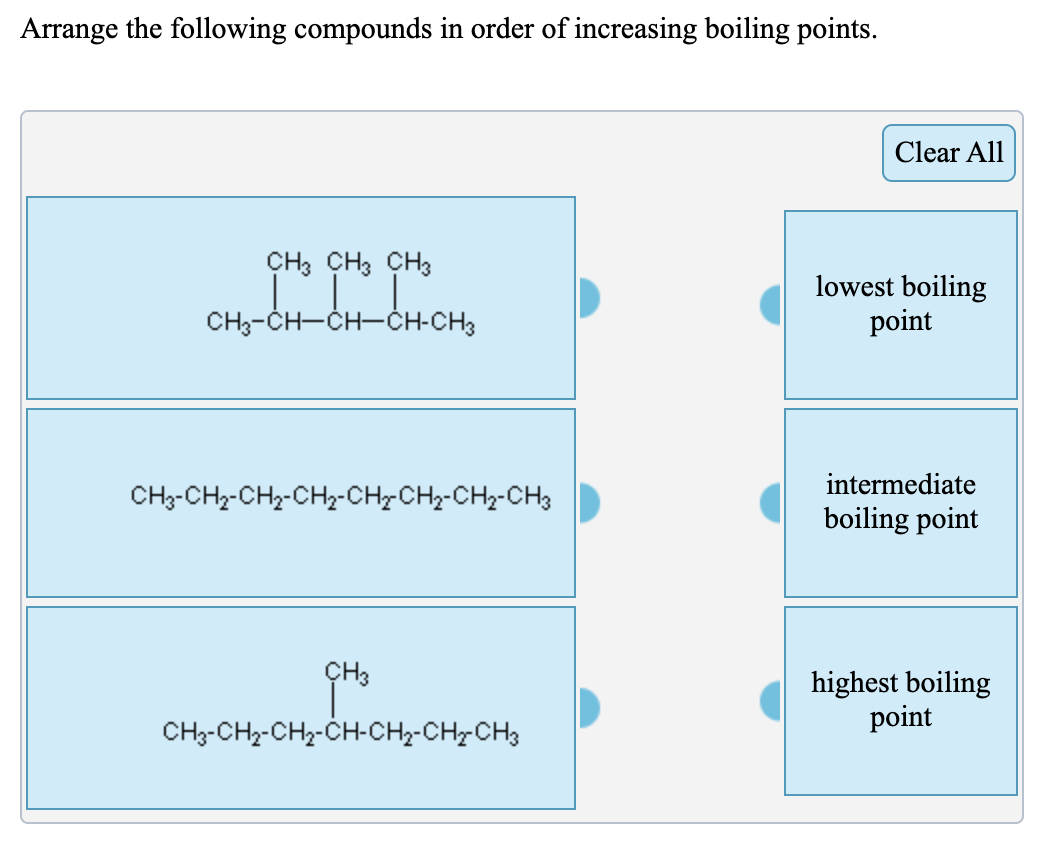
Solved Arrange The Following Compounds In Order Of Chegg Com

Solved Arrange The Following Compounds In Order Of Chegg Com

Arrange The Following Sets Of Compounds In Order Of Their Increasing Boiling Points A Pentan Youtube
Comments
Post a Comment